The Pfizer-BioNTech Covid-19 vaccine, which touts a 95-percent efficacy rate, may not be the most ideal in the Philippine setting, a Thomasian immunology expert has warned.
Meeting the Pfizer vaccine’s storage requirement of -75°C alone may be more difficult than the actual procurement of the vaccine, said Asst. Prof. Jose Francis Abrantes, a microbiologist who teaches immunology and virology at the UST Department of Biological Sciences.
“Where are we going to store those vaccines in that kind of condition? How much more if we are talking about millions of doses to be stored, transported and maintained in Manila where the temperature is for a tropical climate?” he told the Varsitarian.
“We cannot afford to be storing Pfizer because we do not have the resources to do that. We don’t have the infrastructure assuming we could afford it after the mass vaccination,” said Abrantes, who is also a researcher at the Institute for Medical Research in Malaysia.
Health Secretary Francisco Duque III earlier admitted in an interview over ANC that the Philippines did not have the suitable storage facilities for the Pfizer vaccine, but said that the vaccine manufacturer might supply the country with the necessary storage equipment.
Herd immunity ‘expensive’
Duque said vaccinating 60 million Filipinos would be enough to achieve “herd immunity” in the country.
Herd immunity happens when a population becomes immune to a disease once a certain threshold number of the people is vaccinated, according to the World Health Organization.
But achieving herd immunity using the Pfizer vaccines will be impractical, said Abrantes.
“The price is not cheap. [Two vaccine doses cost] P2379. We cannot afford economy-wise paying that amount particularly if we are to vaccinate 60 to 70 percent of our population,” he said.
Abrantes also compared the cost of the Pfizer vaccine to AstraZeneca’s based on data provided by Senate Committee on Finance Chair Sen. Juan Edgardo “Sonny” Angara.
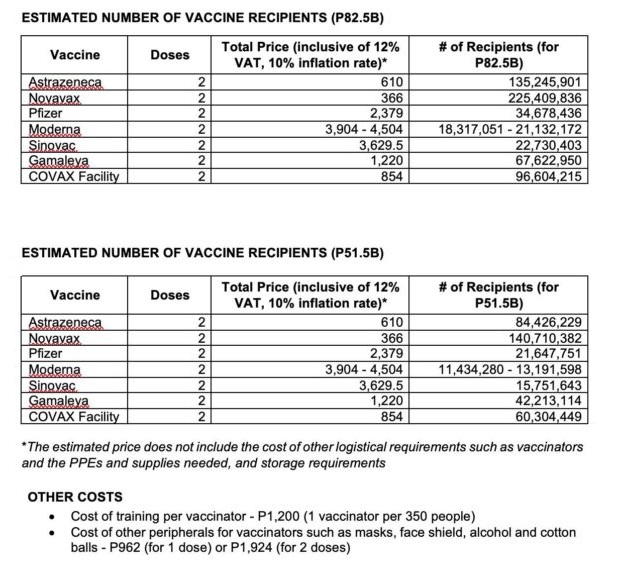
The Philippine government allotted P82.5 billion for Covid-19 vaccines in its 2021 national budget. The amount would cover the vaccination of 34,678,436 recipients if solely Pfizer vaccines were to be used—just around half the threshold to achieve herd immunity.
Two doses of AstraZeneca’s vaccines cost P610. Abrantes noted that the AstraZeneca vaccine, unlike Pfizer’s, could be stored at regular refrigeration temperatures.
“It will require only the normal refrigeration temperature of between 2 to 8°C and that is the most practical in our current situation even for rural areas,” he said.
The government’s P82.5-billion budget would cover the vaccination of more than 135 million Filipinos if solely AstraZeneca vaccines were to be used.
AstraZeneca vaccines were found to have a 70-percent efficacy rate based on interim data.
On Jan. 6, Food and Drug Administration Chief Eric Domingo said AstraZeneca had applied for EUA in the Philippines, joining Pfizer-BioNTech as the only vaccine manufacturers to have applied for EUA in the country.
Last Nov. 27, the country purchased 2.6 million doses of the AstraZeneca Covid-19 vaccine via a tripartite agreement with the private sector for delivery in June 2021.
The Sinovac vaccine from China, which the country is looking to procure 25 million doses of by March 2021, is priced at P3,629.50 for two doses, the second most expensive vaccine based on Senate data.
Several lawmakers have raised concerns about the 50-percent efficacy rate of Sinovac with Sen. Juan Miguel Zubiri calling the Chinese vaccine “a joke” in the fight against the coronavirus.
Abrantes urged health authorities to prioritize Covid-19 vaccines that are cheaper and with better efficacy rates.
“You don’t argue about the political implications of [the Covid-19 vaccines], but just by looking, common sense will tell you that you have to go for the much more effective vaccines at [lower prices],” he said.
Single-dose vaccines preferred
Compliance in vaccination procedures could also pose a challenge, so the country should also look into procuring single-dose vaccines such as the one from Janssen Pharmaceutica, Abrantes said.
“Two doses are already a bit difficult for Filipinos, you know our countrymen, we are very pasaway. I think we should as much as possible choose a [single-dose vaccine], that would be better,” he said.
On Dec. 29, the FDA announced that it had approved Janssen Pharmaceutica’s application to conduct late-stage clinical trials in the Philippines—a first.
The FDA on Jan. 6 said its decision on Pfizer’s EUA request might be released on Jan. 14. K.A.L. Escarilla